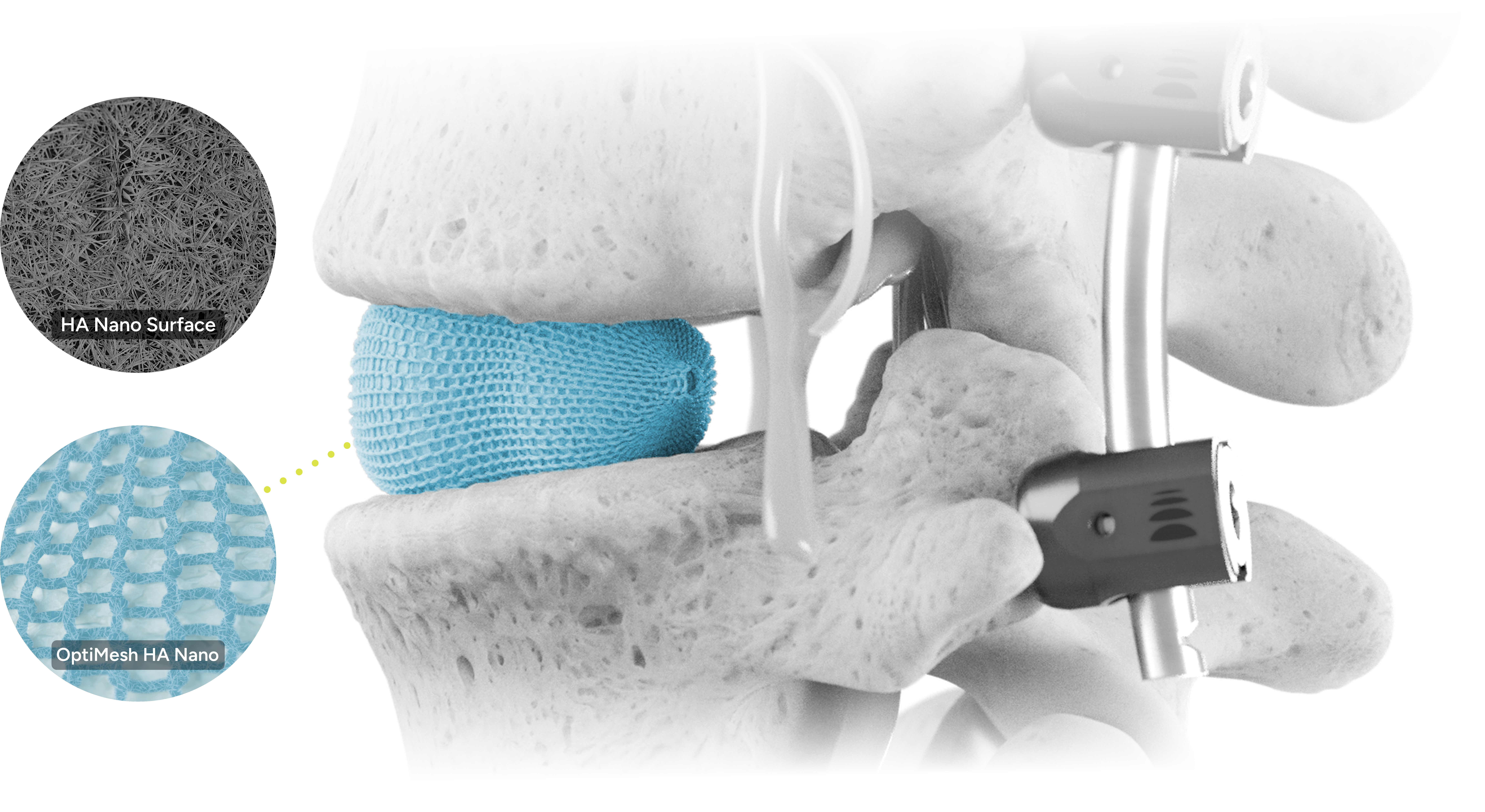
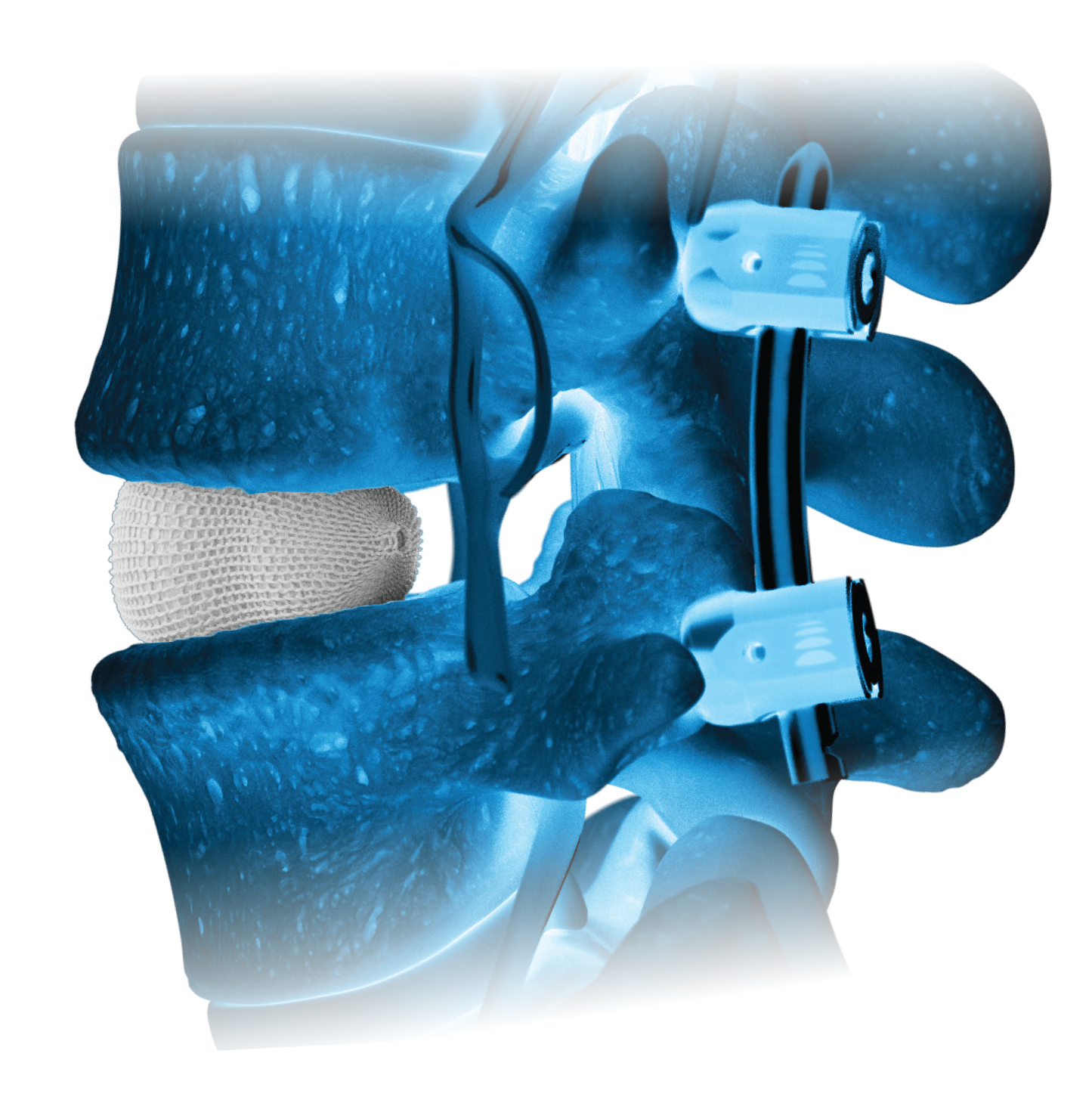
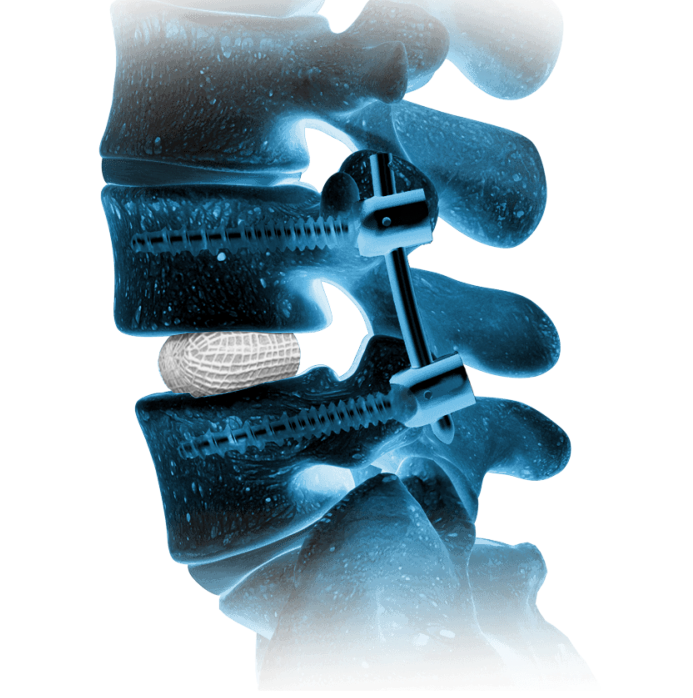
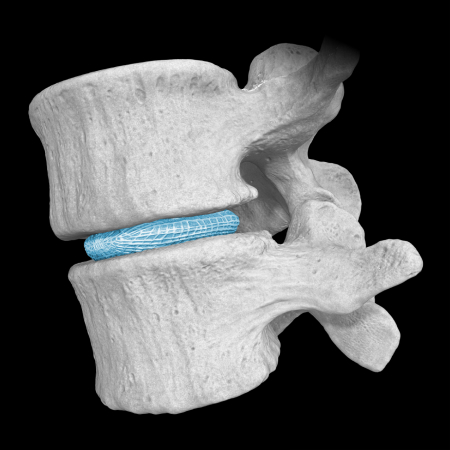
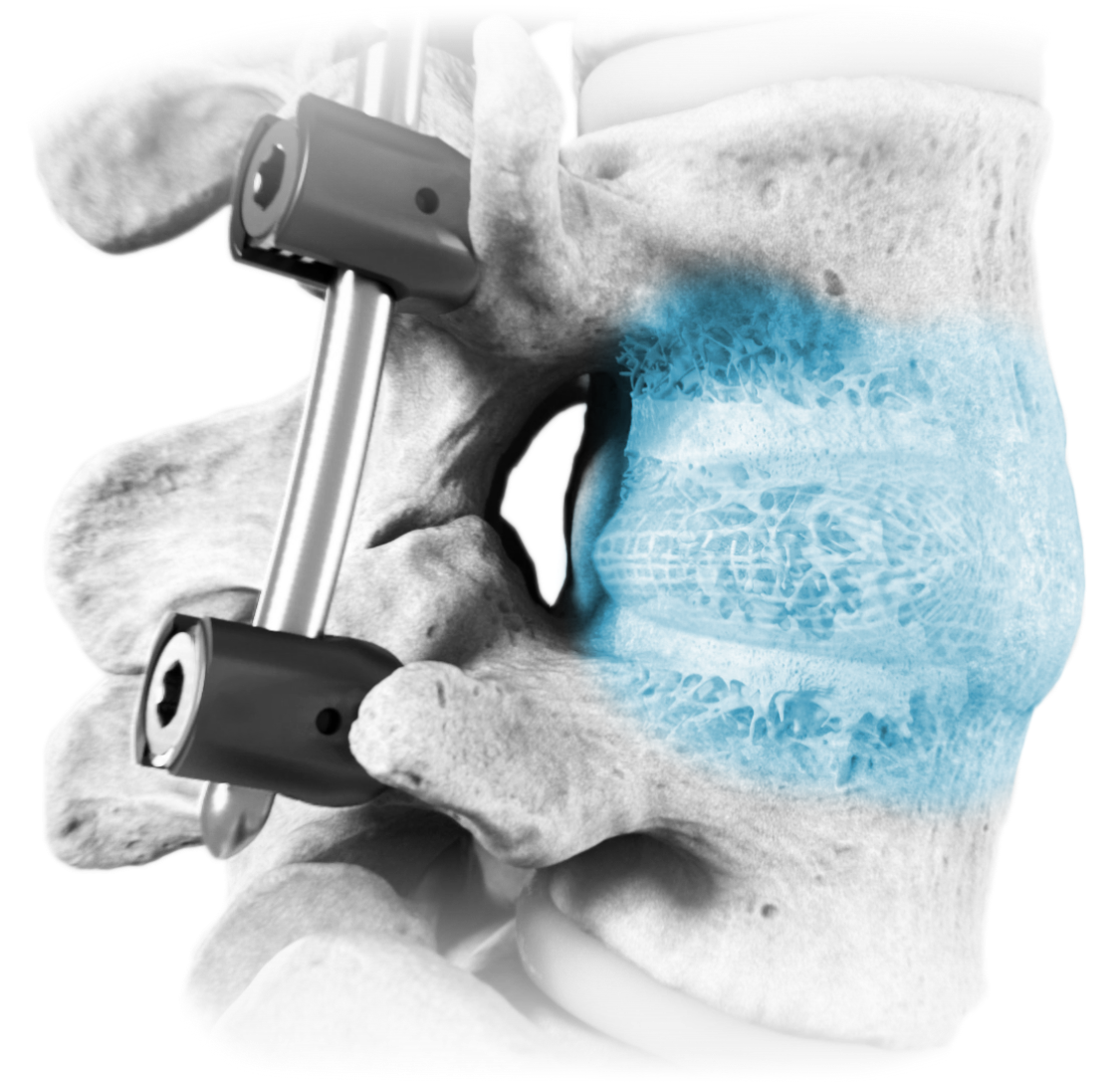
Natural Restoration and Expansion Power
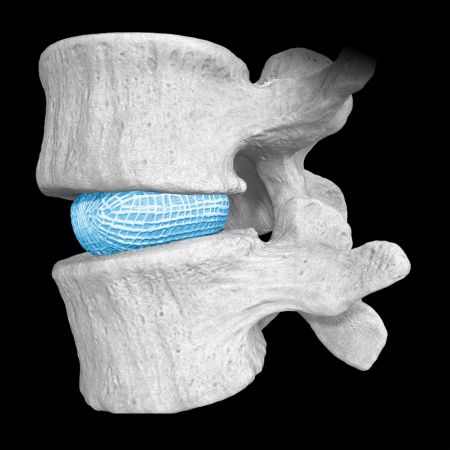
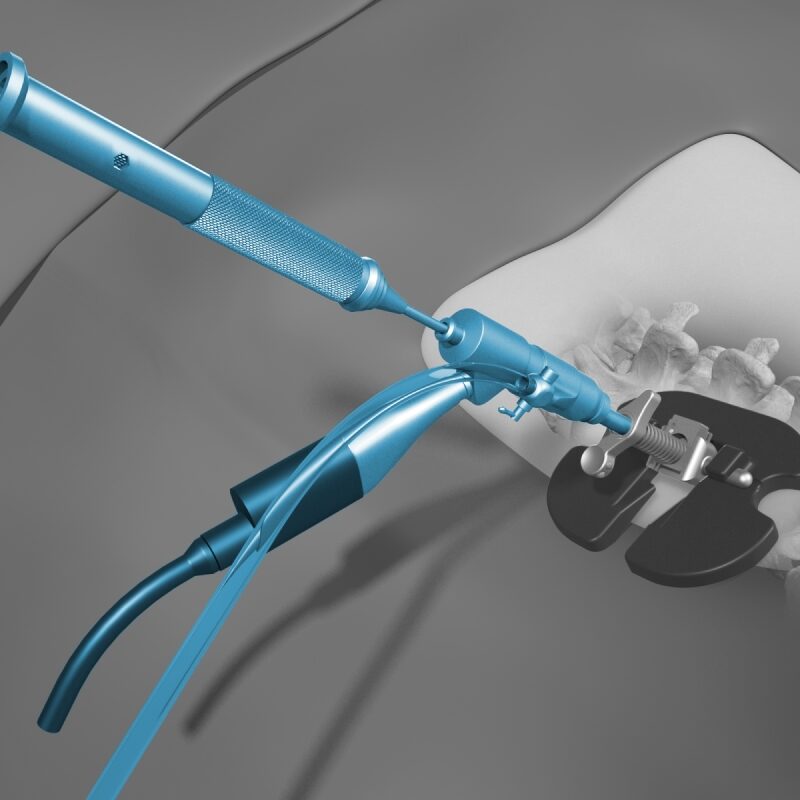
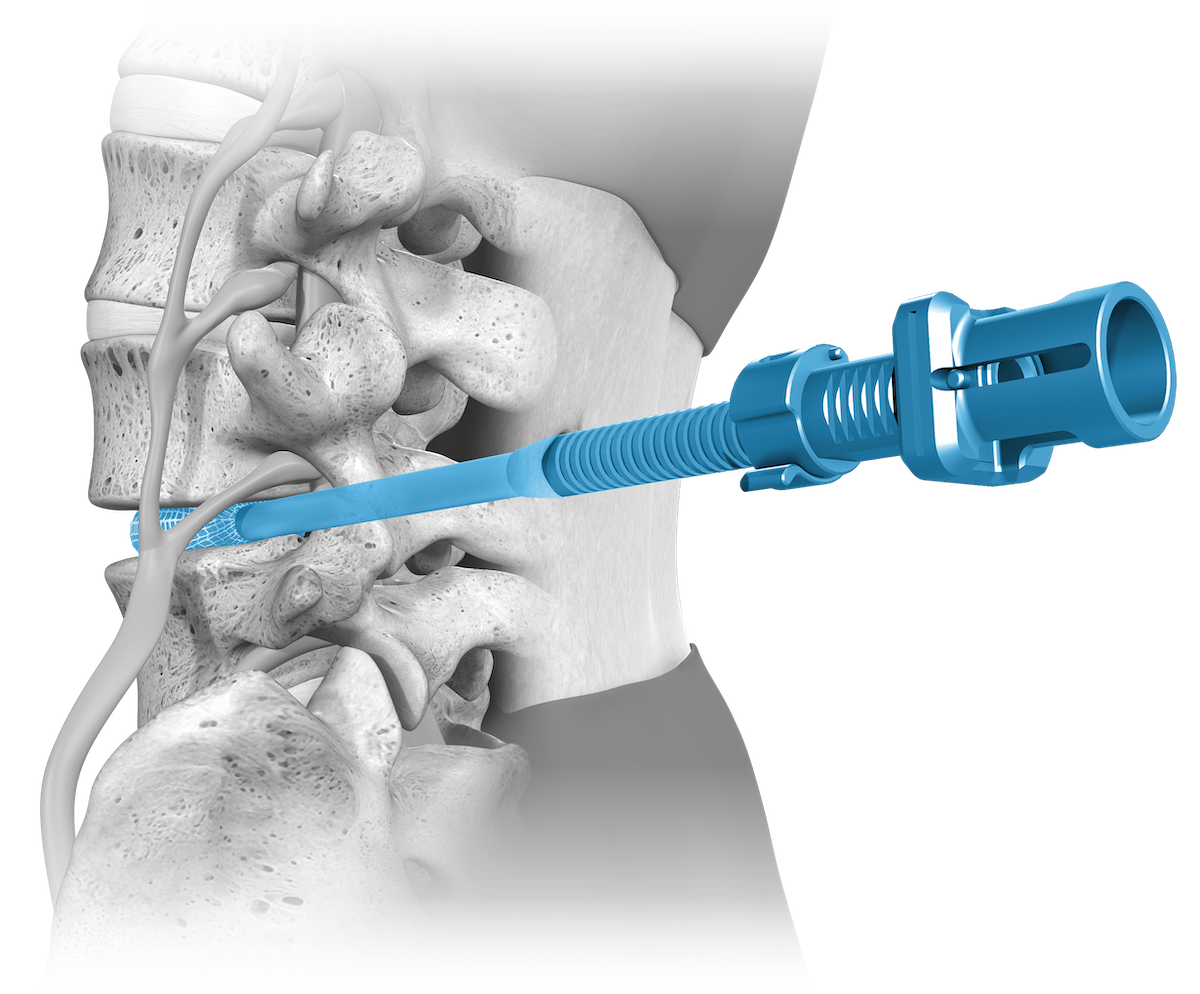
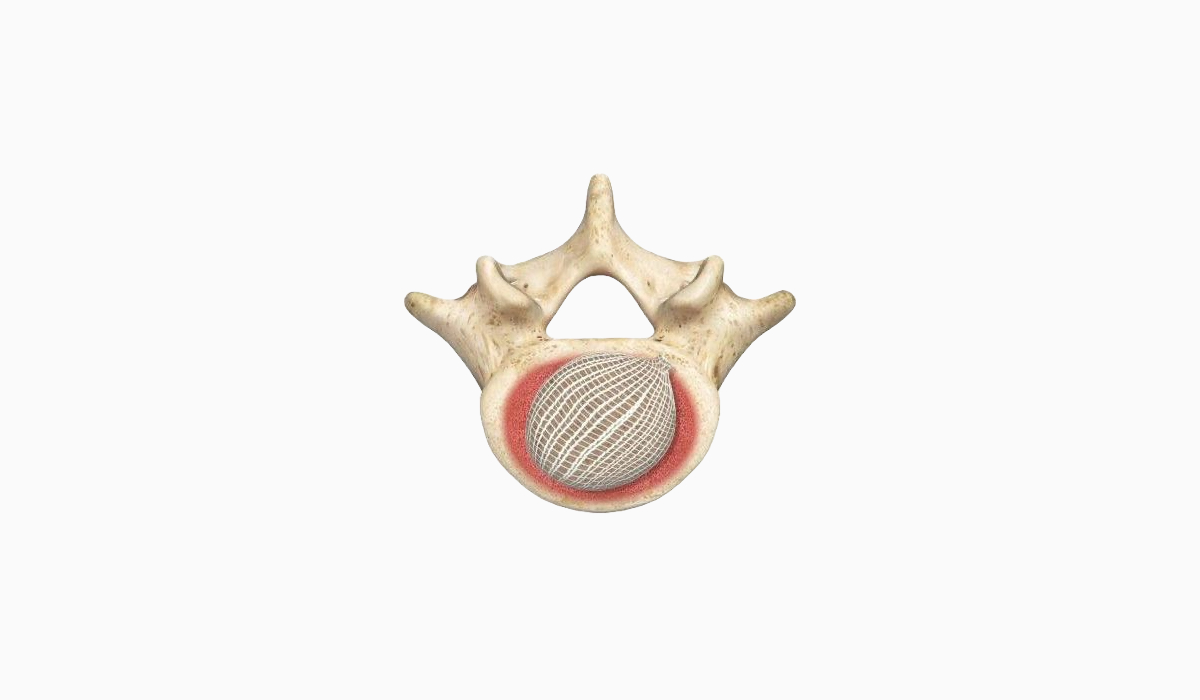
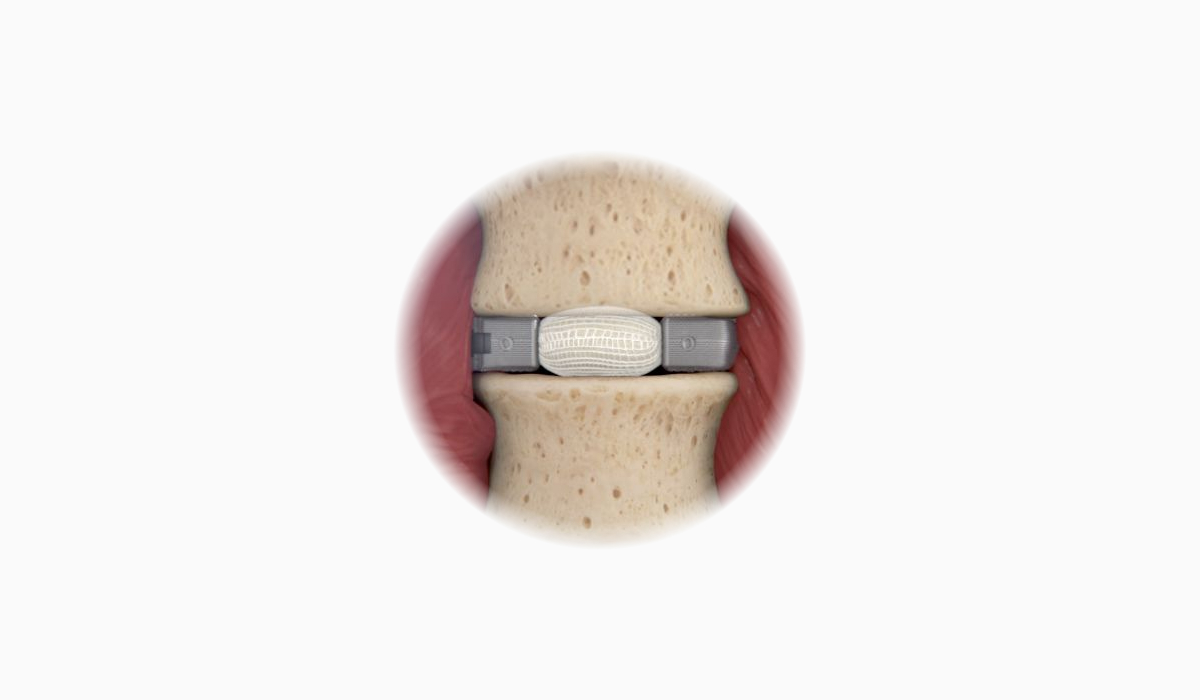
OptiMesh’s powerful distraction forces and conformance to the endplates provide strength and stability to restore disc height, achieve alignment goals, and promote a robust fusion.1
Efficient Delivery of Biologics
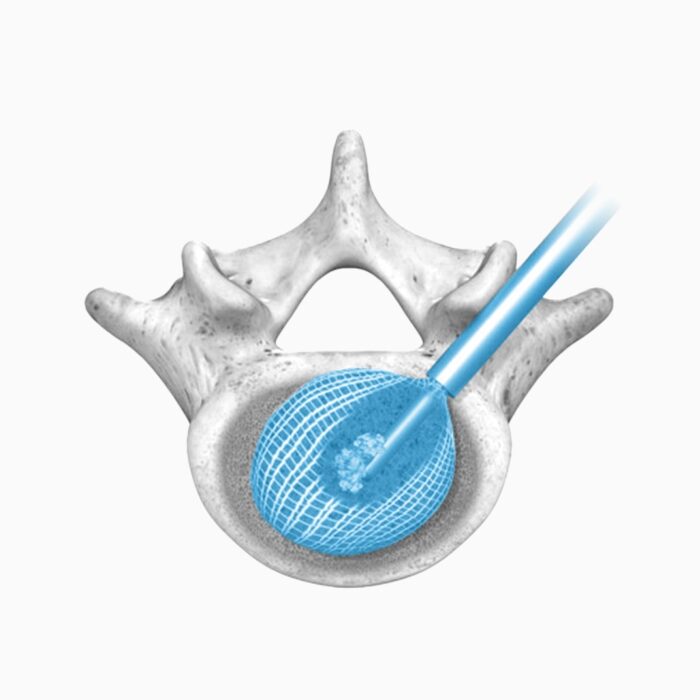
With nearly the entire implant comprised of biologics, OptiMesh offers a comprehensive fusion scaffold and provides one of the most efficient ways to deliver biologics to the spine. Backed with IDE level data, this is an implant category of its own.
Unmatched Clinical Experience
The SCOUT IDE Study
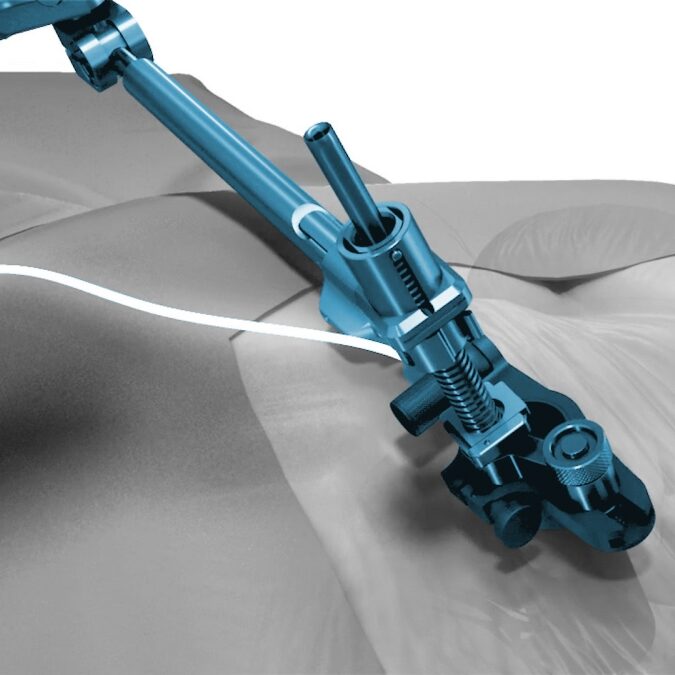
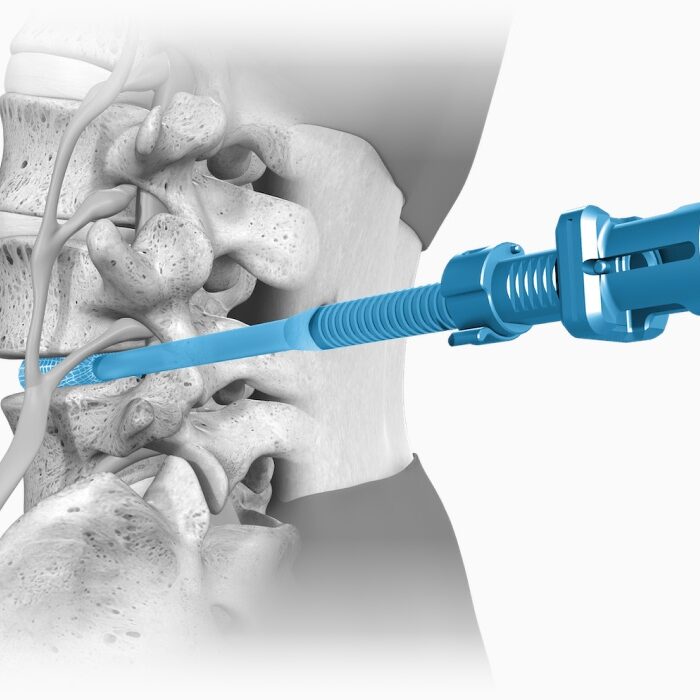
Unique in design and in published data, OptiMesh holds the highest achievement in clinical validation with an IDE De Novo level grant. Spineology Clinical Outcomes Trial (SCOUT Study): IDE trial of a novel, conformable device placed in the disc space through a small portal and filled with bone graft in-situ. At 24 months:
0%
98% fusion at 12 months post-op, evaluated by two independent radiologists.0%
85% of patients experienced a clinically meaningful reduction in back pain at 3 months.0%
75% of patients experienced a clinically meaningful improvement in back function at 3 months.0%
92% of patients rated their satisfaction "excellent" or "good" at 6 months.Chi J, Nunley P, et al. Two-Year Outcomes From a Prospective Multicenter Investigation Device Trial of a Novel Conformal Mesh Interbody Fusion Device. Int J Spine Surg. 2021 Dec; 15(6): 1103-1114.

Unique in Data
20 Clinical Publications
> 50,000 implants
Additionally, 20+ years of clinical experience, 20 clinical publications, and > 50,000 devices implanted set OptiMesh alone in the interbody fusion space.
Spineology
In the News
Read the most important updates and news about Spineology® in the following posts, then click to learn more about us and our growing product and team.